The pharmaceutical industry stands at the precipice of a revolutionary transformation as artificial intelligence emerges as the most powerful catalyst for accelerating drug discovery and development processes that have remained fundamentally unchanged for decades. Traditional drug development pathways, characterized by extensive timelines spanning fifteen to twenty years and costs exceeding several billion dollars per approved medication, are being dramatically reimagined through the application of sophisticated machine learning algorithms, deep neural networks, and computational modeling techniques that promise to reshape the entire landscape of medical innovation.
Discover the latest AI breakthroughs in healthcare and witness how cutting-edge technologies are transforming medical research and patient care across the globe. The convergence of artificial intelligence with pharmaceutical research represents not merely an incremental improvement in existing methodologies, but rather a fundamental paradigm shift that is redefining how we approach the identification, validation, and optimization of therapeutic compounds in the pursuit of more effective treatments for complex diseases.
The Traditional Drug Discovery Challenge
The conventional pharmaceutical development process has long been plagued by extraordinary complexity, astronomical costs, and prohibitively lengthy timelines that have created significant barriers to bringing life-saving medications to patients in need. The traditional pathway from initial compound identification through clinical trials to regulatory approval involves numerous sequential phases, each fraught with substantial risks of failure and requiring enormous investments in both time and financial resources that often exceed the capabilities of all but the largest pharmaceutical corporations.
This conventional approach typically begins with target identification and validation, progresses through extensive compound screening and optimization phases, advances through preclinical testing in laboratory and animal models, and culminates in multiple phases of human clinical trials before regulatory submission and approval. Each stage of this process presents unique challenges, from the initial difficulty of identifying promising molecular targets to the complex task of optimizing compound properties for safety, efficacy, and manufacturability, ultimately resulting in success rates that hover around a mere ten percent for compounds entering clinical development.
The astronomical costs associated with traditional drug development, often estimated between two and three billion dollars per approved medication, reflect not only the direct expenses of research and development activities but also the substantial investments in failed compounds that never reach the market. These economic realities have created a situation where pharmaceutical innovation has become increasingly concentrated among a small number of large corporations with the financial resources to sustain such massive investments, potentially limiting the diversity of research approaches and therapeutic targets pursued.
AI-Powered Target Identification and Validation
Artificial intelligence has revolutionized the initial stages of drug discovery by enabling researchers to identify and validate molecular targets with unprecedented speed and precision through the analysis of vast datasets encompassing genomic information, proteomic profiles, and clinical phenotypes that would be impossible to process using traditional analytical methods. Machine learning algorithms can now analyze complex biological networks, identify previously unknown relationships between genes and diseases, and predict which molecular targets are most likely to yield successful therapeutic interventions.
Advanced AI systems utilize sophisticated pattern recognition capabilities to analyze multi-omics data, integrating information from genomics, transcriptomics, proteomics, and metabolomics to create comprehensive maps of disease mechanisms and identify novel therapeutic opportunities that might be overlooked by conventional research approaches. These systems can process enormous volumes of biomedical literature, clinical databases, and experimental results to identify patterns and correlations that provide insights into disease pathways and potential intervention points.
Experience the power of advanced AI analysis with Claude for complex data interpretation and research acceleration that transforms how we understand biological systems and disease mechanisms. The application of natural language processing techniques to biomedical literature enables AI systems to extract relevant information from millions of scientific publications, creating comprehensive knowledge graphs that can reveal previously unknown connections between seemingly unrelated biological phenomena.
Accelerating Compound Discovery and Design
The application of artificial intelligence to compound discovery and molecular design has fundamentally transformed how researchers approach the identification and optimization of potential drug candidates, enabling the exploration of vast chemical spaces that would be prohibitively expensive and time-consuming to investigate through traditional experimental methods. AI-powered molecular design platforms can generate novel chemical structures with desired properties, predict their biological activity, and optimize their characteristics for specific therapeutic applications.
Generative adversarial networks and other advanced machine learning architectures have enabled the creation of sophisticated molecular design tools that can propose entirely new chemical entities based on desired target profiles, safety characteristics, and pharmacokinetic properties. These systems can explore chemical spaces containing billions or even trillions of potential compounds, identifying promising candidates that meet specific criteria for further development and optimization.
Deep learning models trained on extensive databases of chemical structures and biological activity data can predict how modifications to molecular structures will affect their therapeutic properties, enabling researchers to optimize compounds more efficiently and reduce the number of synthesis and testing cycles required to develop effective drug candidates. This predictive capability significantly accelerates the lead optimization process and reduces the resources required to identify promising compounds for further development.
Revolutionizing Preclinical Testing and Safety Assessment
Artificial intelligence has transformed preclinical testing and safety assessment processes by enabling more accurate prediction of drug toxicity, metabolic behavior, and pharmacokinetic properties through sophisticated computational models that can simulate biological processes with remarkable fidelity. These AI-powered systems can predict potential safety issues, drug-drug interactions, and adverse effects before compounds enter expensive and time-consuming animal studies or human trials.
Machine learning models trained on extensive databases of pharmacological and toxicological data can identify potential safety concerns that might not be apparent through traditional testing methods, enabling researchers to make informed decisions about compound progression much earlier in the development process. This predictive capability not only reduces the risk of late-stage failures but also minimizes the use of animal testing by providing reliable alternatives for initial safety screening.
AI systems can also optimize dosing strategies, predict patient responses to different treatment regimens, and identify biomarkers that can be used to monitor drug efficacy and safety in clinical settings. These capabilities enable more personalized approaches to drug development and clinical testing, potentially improving success rates and reducing the time required to demonstrate therapeutic benefit.
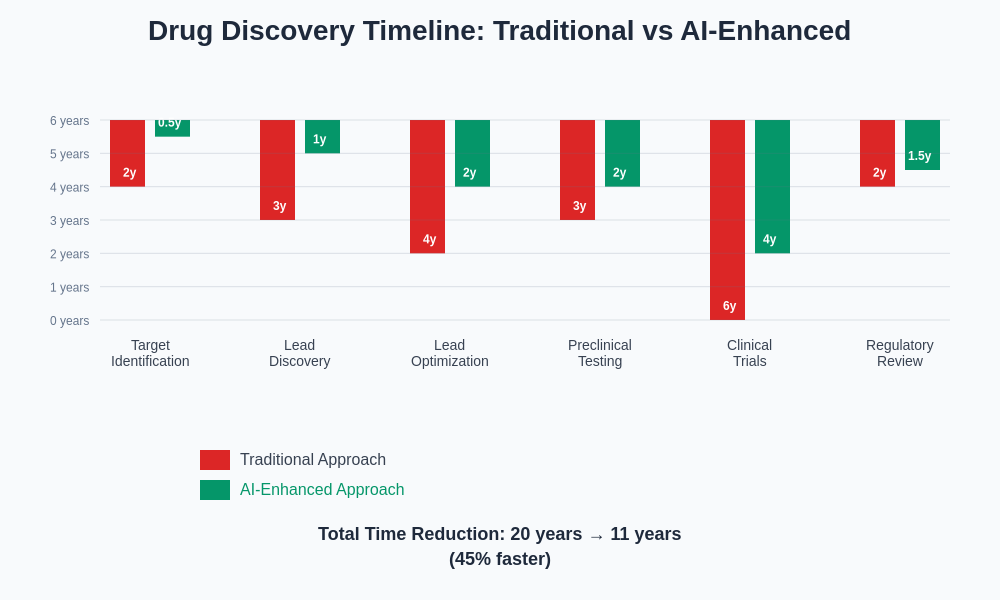
The transformation of drug discovery timelines through AI integration demonstrates dramatic reductions in development phases that traditionally consumed years of research and development resources. This acceleration enables pharmaceutical companies to bring life-saving treatments to patients more rapidly while reducing overall development costs and improving the economics of drug development.
Enhancing Clinical Trial Design and Patient Recruitment
The application of artificial intelligence to clinical trial design and patient recruitment has addressed some of the most significant challenges in pharmaceutical development, enabling more efficient trial protocols, improved patient stratification, and enhanced prediction of trial outcomes that can significantly reduce the time and cost associated with clinical development phases. AI systems can analyze vast amounts of clinical data to identify optimal trial designs, predict patient responses, and optimize recruitment strategies.
Machine learning algorithms can analyze electronic health records, genomic data, and clinical databases to identify patients who are most likely to benefit from specific treatments, enabling more targeted recruitment strategies and improved trial efficiency. This precision in patient selection not only increases the likelihood of demonstrating therapeutic benefit but also reduces the number of patients required for trials and accelerates enrollment timelines.
AI-powered systems can also monitor clinical trials in real-time, identifying potential safety signals, efficacy trends, and protocol deviations that enable rapid response to emerging issues and optimization of trial conduct. This continuous monitoring capability can prevent costly trial failures and enable adaptive trial designs that can be modified based on accumulating evidence of safety and efficacy.
Leverage Perplexity’s research capabilities for comprehensive analysis of clinical trial data and pharmaceutical research trends that inform strategic decision-making in drug development. The integration of AI into clinical development processes enables pharmaceutical companies to make data-driven decisions about trial continuation, protocol modifications, and regulatory strategies.
Transforming Drug Repurposing and Combination Therapy
Artificial intelligence has opened new avenues for drug repurposing and combination therapy development by enabling researchers to identify novel therapeutic applications for existing compounds and predict synergistic effects between different drugs through comprehensive analysis of molecular mechanisms, clinical data, and biological networks. This approach can significantly reduce development timelines and costs by leveraging existing safety and pharmacological data.
AI systems can analyze vast databases of approved drugs, their molecular targets, and clinical effects to identify potential new therapeutic applications that might not be apparent through traditional research approaches. Machine learning algorithms can predict how existing drugs might interact with disease pathways in different therapeutic contexts, enabling researchers to rapidly identify promising repurposing opportunities.
The development of combination therapies, which has traditionally relied on empirical approaches and extensive experimental testing, has been revolutionized through AI systems that can predict drug interactions, optimize dosing strategies, and identify synergistic combinations that maximize therapeutic benefit while minimizing adverse effects. These predictive capabilities enable more rational approaches to combination therapy development and reduce the extensive experimental work traditionally required to optimize multi-drug regimens.
Personalized Medicine and Precision Drug Development
The integration of artificial intelligence with genomic medicine and precision healthcare has enabled the development of personalized drug discovery approaches that can identify therapeutic strategies tailored to individual patient characteristics, genetic profiles, and disease subtypes. This personalized approach to drug development represents a fundamental shift from the traditional one-size-fits-all model toward more targeted and effective therapeutic interventions.
AI systems can analyze patient genomic data, biomarker profiles, and clinical characteristics to predict individual responses to specific treatments, enabling the development of companion diagnostics and personalized treatment protocols that maximize therapeutic benefit while minimizing adverse effects. This precision approach not only improves patient outcomes but also enhances the efficiency of clinical development by enabling more targeted patient populations and improved success rates.
Machine learning algorithms can identify patient subgroups that are most likely to respond to specific treatments, enabling stratified clinical trial designs and regulatory approval strategies that focus on populations most likely to benefit from new therapies. This approach can significantly improve the probability of clinical success and enable more efficient allocation of development resources.
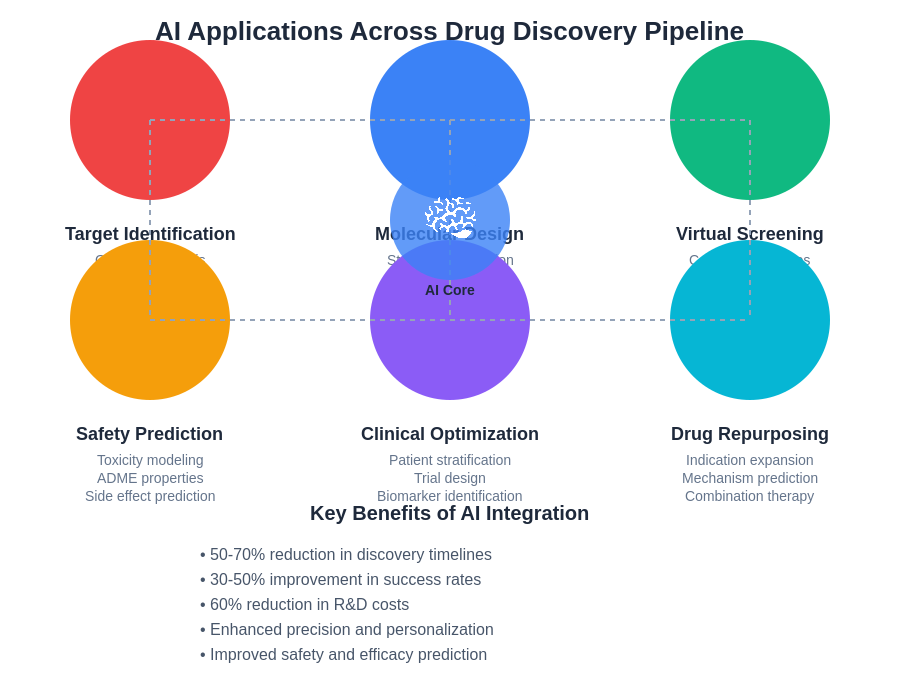
The diverse applications of artificial intelligence across the drug discovery pipeline demonstrate the comprehensive transformation of pharmaceutical research, from initial target identification through clinical development and regulatory approval. Each application area contributes to accelerating the overall development process while improving the quality and success rate of new therapeutic interventions.
Regulatory Considerations and Validation Challenges
The integration of artificial intelligence into drug discovery and development processes has raised important questions about regulatory oversight, validation requirements, and quality assurance standards that must be addressed to ensure the safety and efficacy of AI-developed therapeutics. Regulatory agencies worldwide are developing new frameworks and guidance documents to address the unique challenges posed by AI-driven drug development approaches.
The validation of AI models used in drug discovery requires careful consideration of data quality, model transparency, and reproducibility standards that ensure reliable and consistent performance across different applications and datasets. Regulatory agencies are working to establish standards for AI model validation, documentation requirements, and ongoing monitoring protocols that maintain confidence in AI-generated results while enabling innovation in pharmaceutical research.
The explainability of AI systems has become a critical consideration for regulatory acceptance, as regulators require clear understanding of how AI models make predictions and recommendations that influence critical development decisions. This has driven the development of interpretable machine learning approaches and model explanation techniques that can provide insights into AI decision-making processes.
Economic Impact and Industry Transformation
The economic implications of AI-driven drug discovery extend far beyond simple cost savings, encompassing fundamental changes in pharmaceutical industry structure, competitive dynamics, and investment patterns that are reshaping the entire landscape of biomedical innovation. The reduction in development costs and timelines enabled by AI technologies has the potential to democratize drug development and enable smaller organizations to compete with established pharmaceutical giants.
The improved efficiency and success rates associated with AI-driven drug discovery have attracted significant investment from venture capital firms, pharmaceutical companies, and technology companies seeking to capitalize on the transformative potential of these technologies. This investment influx has accelerated the development and adoption of AI tools while fostering innovation in computational approaches to drug discovery.
The transformation of pharmaceutical research through AI has also created new business models and collaborative structures, including partnerships between technology companies and pharmaceutical organizations, AI-focused biotechnology startups, and academic-industry collaborations that leverage complementary expertise and resources. These new models are enabling more efficient allocation of resources and accelerating the translation of AI innovations into clinical applications.
Future Directions and Emerging Technologies
The future of AI-driven drug discovery promises even more dramatic advances through the integration of emerging technologies such as quantum computing, advanced robotics, and next-generation sequencing platforms that will further accelerate pharmaceutical research and enable entirely new approaches to therapeutic development. These technological advances will expand the capabilities of AI systems and enable more sophisticated analysis of biological systems.
Quantum computing applications in drug discovery are expected to enable more accurate molecular simulations, optimization of complex drug interactions, and exploration of larger chemical spaces than currently possible with classical computing approaches. These capabilities could revolutionize our understanding of molecular mechanisms and enable the development of entirely new classes of therapeutic interventions.
The integration of AI with advanced laboratory automation and robotics platforms is creating autonomous drug discovery systems that can design, synthesize, and test compounds with minimal human intervention. These integrated systems promise to further accelerate the drug discovery process while reducing costs and improving reproducibility of experimental results.
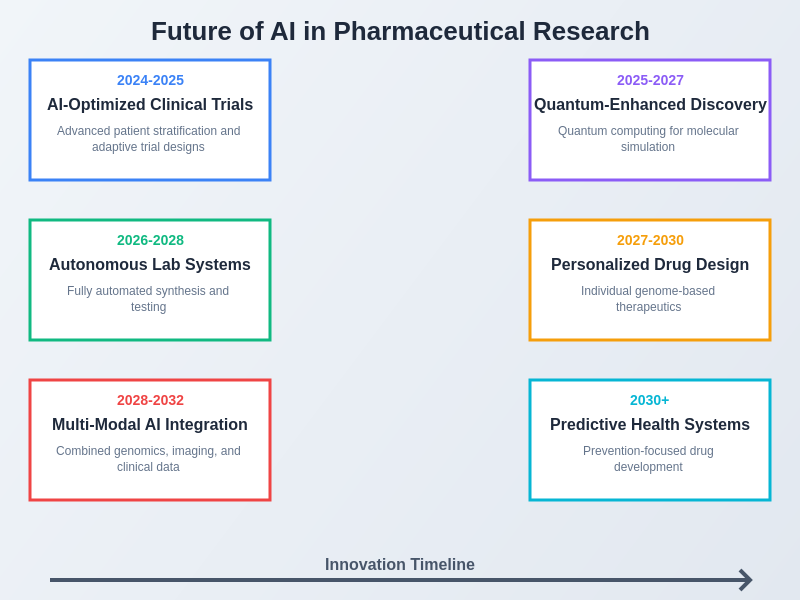
The convergence of multiple advanced technologies with artificial intelligence is creating unprecedented opportunities for pharmaceutical innovation, promising to address some of the most challenging diseases and unmet medical needs through novel therapeutic approaches that were previously inconceivable.
Global Collaboration and Data Sharing Initiatives
The success of AI-driven drug discovery increasingly depends on large-scale collaboration and data sharing initiatives that bring together diverse stakeholders including pharmaceutical companies, academic institutions, regulatory agencies, and patient organizations to create comprehensive datasets and shared resources that benefit the entire research community. These collaborative efforts are essential for maximizing the potential of AI technologies in pharmaceutical research.
International consortiums and public-private partnerships have emerged to facilitate data sharing, standardization of research protocols, and development of common AI platforms that can be utilized across different organizations and research programs. These initiatives help overcome the data silos that have traditionally limited the effectiveness of AI applications in drug discovery while ensuring that the benefits of technological advances are broadly distributed.
The establishment of shared databases, standardized data formats, and common analytical platforms has enabled more effective collaboration between research organizations and accelerated the development of AI tools that can be validated and applied across different research contexts. These collaborative infrastructure investments are essential for realizing the full potential of AI in pharmaceutical research.
Ethical Considerations and Patient Access
The implementation of AI-driven drug discovery raises important ethical questions about patient access to innovative treatments, data privacy and security, and the responsibility of pharmaceutical companies to ensure that AI-developed therapies are accessible to patients who need them regardless of their economic circumstances or geographic location. These considerations require careful attention to ensure that AI advances benefit all patients equitably.
The use of patient data in AI model development raises questions about informed consent, data ownership, and privacy protection that must be addressed through robust governance frameworks and ethical oversight mechanisms. Pharmaceutical companies and research organizations must implement comprehensive data protection measures while ensuring that patients retain control over how their information is used in research applications.
The potential for AI to reduce drug development costs and accelerate therapeutic innovation creates opportunities to improve patient access to life-saving treatments, but realizing these benefits requires thoughtful consideration of pricing strategies, distribution mechanisms, and global health equity considerations that ensure that AI advances contribute to improved health outcomes for all populations.
Conclusion and Industry Outlook
The integration of artificial intelligence into drug discovery and pharmaceutical research represents one of the most significant technological transformations in the history of medicine, with the potential to dramatically accelerate the development of life-saving treatments while reducing costs and improving success rates across the entire pharmaceutical industry. The continued advancement of AI technologies, coupled with growing industry adoption and regulatory support, promises to unlock unprecedented opportunities for therapeutic innovation.
The success of AI-driven drug discovery initiatives across multiple therapeutic areas demonstrates the broad applicability and transformative potential of these technologies, while ongoing investments in research and development continue to expand the capabilities and applications of AI in pharmaceutical research. The convergence of AI with other emerging technologies promises even more dramatic advances in the years ahead.
The future of pharmaceutical research lies in the intelligent integration of human expertise with artificial intelligence capabilities, creating collaborative environments where human creativity and scientific knowledge are augmented by the computational power and analytical capabilities of advanced AI systems. This partnership between human intelligence and artificial intelligence represents the most promising pathway toward addressing the world’s most challenging health problems through innovative therapeutic interventions.
Disclaimer
This article is for informational purposes only and does not constitute medical or investment advice. The views expressed are based on current understanding of AI technologies and their applications in pharmaceutical research. Readers should consult with qualified professionals before making decisions related to healthcare investments or treatment options. The effectiveness and regulatory approval of AI-developed therapies may vary, and all medical treatments should be discussed with qualified healthcare providers.
